Whether you are building a new plant, renovating, or extending your current operations, the first step in implementing a high performing cGMP modular facility starts with review/design of your engineering process.
15 years’ experience in Process Engineering for the Life sciences industry
You may have your own team dedicated to process engineering. However, at C-CUBE we benefit from 15 years’ experience in designing processes in the pharmaceutical and biotech industries thanks to our Swiss partner, SP Groups.
Their multi-skilled teams are able to bring to your project the most innovative process engineering knowledge and best practice from Europe.
Gain time in your facility design
Handling both process engineering and facility design will cut into the time allocated to conceptual design phase.
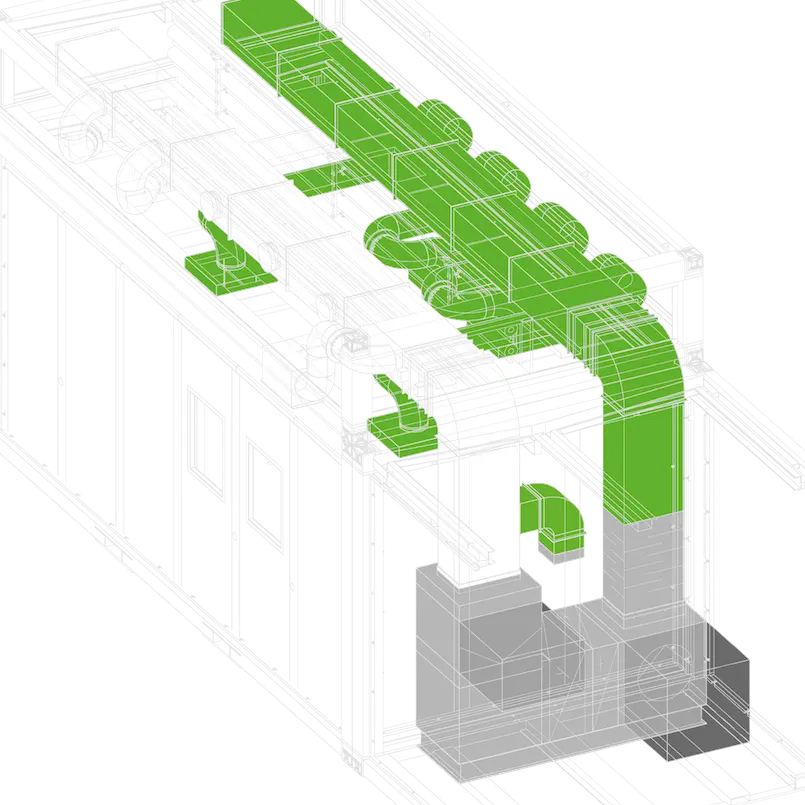
At C-CUBE, facilities are based on an assembly of prefabricated standard modules (in three different sizes).
Our modular concept has been pre-engineered reducing the design phase, with a focus on efficiency, compliance and scalability. The engineering team use BIM to flawlessly fast track the design of your project.

C-CUBE’s competency in
design, build and
qualification are key
to a cost-effective and
successful cGMP facility
project implementation.
If you would like to discuss the feasibility of a modular
construction for any of your applications
Increase the predictability of your outcomes
Process engineering and construction design knowledge combined with a pre-engineered and tested solution will greatly increase predictability and reduce risk for your project.
By end of design phase you will get our strong commitment on:
Our modular concept includes three standard modules. We can build any type of life sciences facility, from pilot scale units to full production facilities… The possibilities are endless.
95% of project construction is completed in a fully controlled, off-site, industrial manufacturing environment. Essentially, the cost is predictable and your budget is secure. Furthermore, modular constructions are more competitive compared to conventional ones.
The industrial parallel manufacturing approach reduces the possibility for over runs. We can precisely detail each phase of the construction process and mitigate lead-time by 50% compared to traditional construction methods.

C-CUBE brings together 15
years of experience in the
pharmaceutical industry to
innovate the life sciences
facility of tomorrow